Overview
Precision Medicine marks a transformative era in healthcare to revolutionize disease prevention, diagnosis, and treatment. Realizing this vision calls for paradigm-shifting analytical tools and robust biomarkers for population screening, early cancer diagnosis, and personalized therapy. Working at the nexus of chemistry, nanomaterials, bioengineering, and medicine, our group strives to develop innovative micro/nanoscale technologies to tackle pressing clinical challenges, including cancer, infectious diseases, and neurodegenerative diseases. Our innovations span advanced bioassays, lab-on-a-chip systems, and next-generation biosensing modalities empowered by CRISPR/Cas systems, nanobiohybrids, robotics and artificial intelligence (AI). We aim to create new analytical capabilities for the quantitative, sensitive, and accurate measurement of diverse biomarkers and liquid biopsies, including proteins, nucleic acids, glycans, exosomes, and tumor cells. In close collaboration with clinicians, our long-term interest lies in translational research to advance laboratory innovations into clinical utilities that improve patient outcomes. Our research programs have been continuously supported by the National Institutes of Health and other funding agencies.
Some specific research areas are briefly introduced below. Postdoc scholars, graduate students, and undergraduates interested in our research are encouraged to contact us for more information.
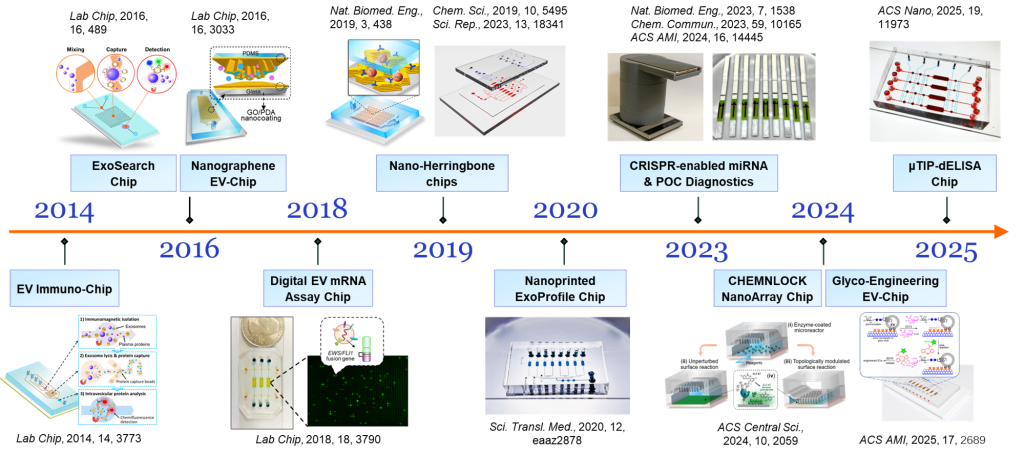
Research Areas
Liquid biopsy Diagnostics
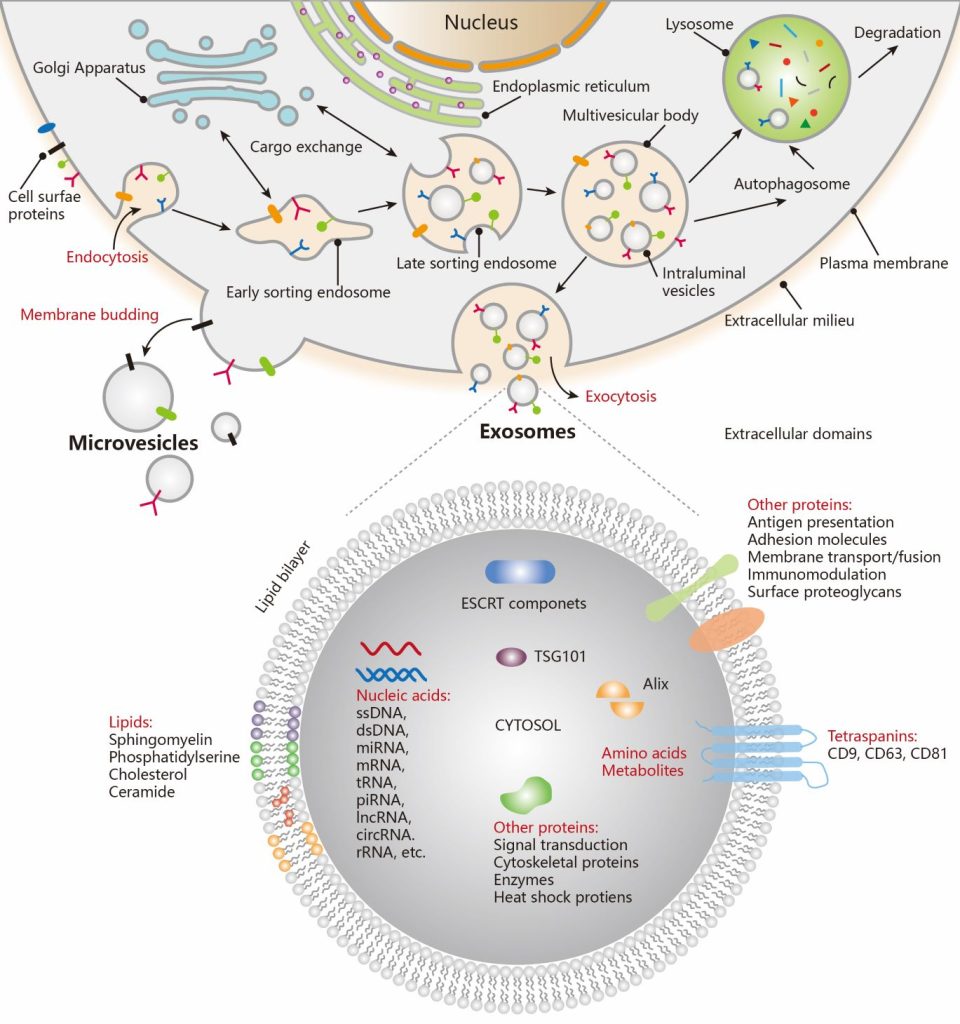
Clinical implementation of Precision Medicine faces major challenges in disease stratification and staging, determining optimal treatment, monitoring therapy response, and overcoming drug resistance and relapse. Exosome research is a new frontier in biomedical sciences and clinical development of cancer diagnostics and therapeutics. Exosomes, membrane vesicles of 30-150 nm in size secreted by most cells, have been identified as a key mediator of many cellular processes including tumor progression, metastasis, and drug resistance. In the past decade, accumulating evidence support exosomal extracellular vesicles (EVs) as an emerging paradigm of “liquid biopsy” that is likely to have significant clinical benefit under situations where early diagnosis and therapy monitoring is particularly challenging. Our lab is focused on developing novel microfluidic systems and nanobiosensing assays that create new capabilities of EV analysis to advance non-invasive liquid biopsy for preclinical screening, early diagnosis, and therapeutic monitoring in cancer. Our long-term goal is to develop and translate transformative strategies for cancer diagnostics and treatment monitoring into clinically useful research and treatment protocols.
Selected Publications:
- Zhang, P. et al. Ultrasensitive quantification of tumor mRNAs in extracellular vesicles with an integrated microfluidic digital analysis chip. Lab on a Chip 2018, 18, 3790–3801.
- Zhang, P. et al. Ultrasensitive detection of circulating exosomes with a 3D-nanopatterned microfluidic chip. Nature Biomedical Engineering 2019, 3, 438–451.
- Zhang, P. et al, Molecular and Functional Extracellular Vesicle Analysis Using Nanopatterned Microchips Monitors Tumor Progression and Metastasis, Science Translational Medicine, 2020, 12, eaaz2878.
- H. Yan, Y. Li, S. Cheng, Y. Zeng, Advance in Analytical Technologies for Extracellular Vesicles, Analytical Chemistry 2021, 93, 4739-4774 (Review).
- Tran, et al., Extracellular Vesicles for Clinical Diagnostics: From Bulk Measurements to Single-Vesicle Analysis, ACS Nano, 2025 19, 31, 28021–28109 (Review).
Digital Chemistry & Biosensing
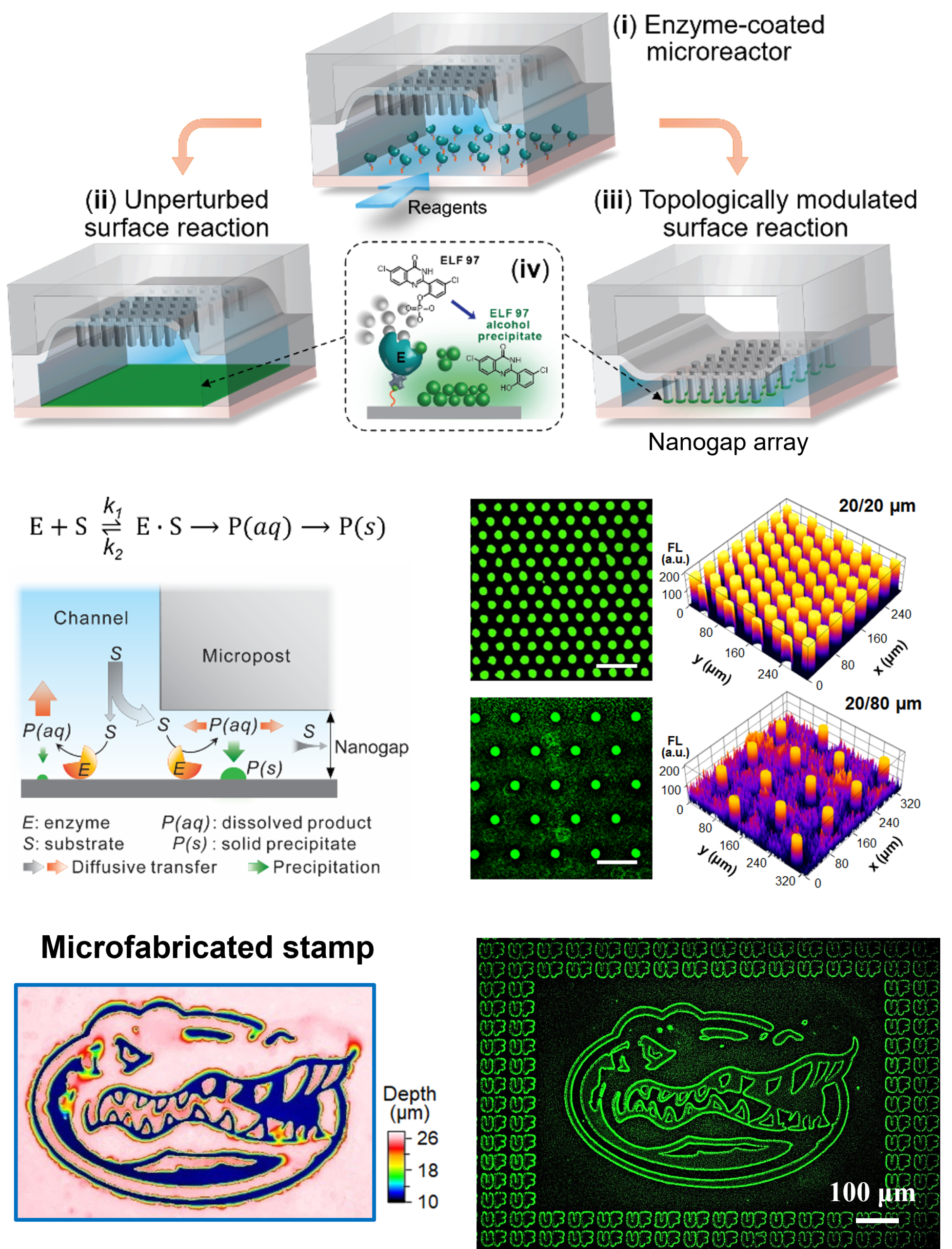
Sensitive and quantitative analysis of low abundant biomolecules is vital to elucidating cell functions and disease pathology and clinical diagnostics. For example, single-cell analysis is necessary to uncover tumor heterogeneity, while the detection of early-stage diseases demands the ability to detect biomarkers present at extremely low concentrations in biofluids (e.g., 10−18 to 10−12 M). To address these challenges, we employ the emerging concept of digital chemistry to develop new enabling bioanalytical tools. In contrast to traditional “analog” approaches, “digital” methods manipulate and measure single molecules in massively parallel, discrete reactions, enabling absolute target counting with single-molecule sensitivity and markedly improved accuracy and reproducibility. Our research in this area focuses on developing innovative micro/nanoscale technologies to interrogate single molecules of nucleic acids and proteins, including enzymes. We are also examining single EVs to elucidate the molecular and functional heterogeneity of EV populations in biofluidis in relation to their biological functions and clinical relevance. Our overarching goal is to understand molecular behaviors and biochemical reactions at the single molecule/vesicle level, and to establish next-generation analytical capabilities for quantitative measurements of complex biological systems.
Selected Publications:
- Y. Wen, et al., Partition-Less Digital Immunoassay Using Configurable Topographic Nanoarrays for Extracellular Vesicle Diagnosis of Ewing Sarcoma, ACS Nano, 2025, 19, 12, 11973–11986.
- Y. Wen, Y. Li, H. Chu, S. Cheng, Y. Zeng, Hydromechanical Modulation of Enzymatic Kinetics Using Microfluidically Configurable Nanoconfinement Arrays, ACS Central Science, 2024, 10 (11), 2059-2071.
- X. Zhou, G.C. Ravichandran, P. Zhang, Y. Zeng, A Microfluidic Alternating-Pull-Push Active Digitization Method for Reproducible Sample-Loss-Free Digital PCR, Lab on a Chip, 2019, 19, 4104–4116.
- P. Zhang, et al., Ultrasensitive Quantification of Tumor mRNAs in Extracellular Vesicles with Integrated Microfluidic Digital Analysis Chip, Lab on a Chip, 2018, 18, 3790–3801.
CRISPR-Empowered Biosensing & Point-of-Care Diagnostics
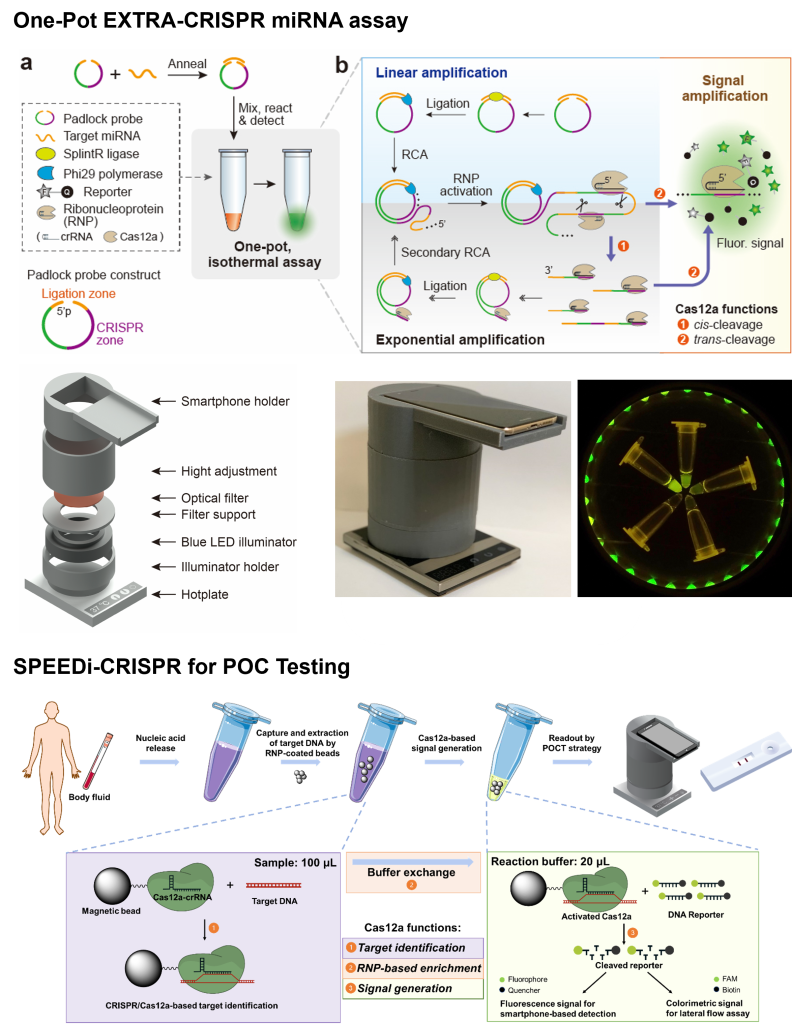
CRISPR (clustered regularly interspaced short palindromic repeats) systems provide powerful tools for gene editing and have recently emerged as a versatile platform for next-generation biosensing assays, combining the analytical performance of PCR with the simplicity of isothermal amplifications. In particular, the CRISPR-Cas12a and Cas13a systems offer highly specific target recognition through Cas-crRNA complexes and enzymatic signal amplification via collateral cleavage of fluorogenic probes by Cas enzyme activated upon target binding. A variety of CRISPR assays have been reported for viral DNA and RNA detection, though most require an additional PCR or isothermal pre-amplification step to achieve desirable sensitivity. Notably, CRISPR-based SARS-CoV-2 viral RNA tests reshaped the landscape of nucleic acid testing during the COVID-19 pandemic, underscoring their clinical potential.
Motivated by these advances, our group has been developing innovative chemistries and engineering approaches to harness CRISPR/Cas systems for disease diagnostics. Radically deviating from the existing strategies, for instance, we recently established a one-step, one-pot isothermal miRNA assay termed “Endonucleolytically eXponenTiated Rolling circle Amplification with dual-functional CRISPR-Cas12a” (EXTRA-CRISPR) which enables rapid and simple miRNA detection with RT-qPCR comparable performance. Our long-term vision is to develop the next-generation, CRISPR-empowered bioassays to promote liquid biopsy-based cancer diagnostics and point-of-care (POC) testing for infectious diseases.
Selected Publications:
- Z. Tian, H. Yan, Y. Zeng, “Solid-Phase Extraction and Enhanced Amplification-Free Detection of Pathogens Integrated by Dual-Functional CRISPR-Cas12a”, ACS Applied Materials & Interfaces, 2024, 16, 12, 14445–14456.
- H. Yan, S. Han, S. Hughes, Y. Zeng, “Extraction-Free, One-Pot CRISPR/Cas12a Detection of MicroRNAs Directly from Extracellular Vesicles”, Chemical Communications, 2023, 59, 10165-10168.
- H. Yan, et al., “A One-pot Isothermal Cas12-based Assay for the Sensitive Detection of MicroRNAs”, Nature Biomedical Engineering, 2023, 7, 1583–1601.
Hybrid Nanobiotics for Intelligent Diagnostics
Hybrid nanobiotics are rapidly emerging as a disruptive paradigm that fuses synthetic nanomaterials, biological moieties, and robotic functionalities into autonomous, adaptive micro/nanoscale systems. Unlike traditional nanobiotechnology which often emphasizes static conjugation of nanomaterials with biomolecular elements, hybrid nanobiotics bridges synthetic engineering with biological adaptability to incorporate dynamic and programmable behaviors, such as autonomous motility, self-organization, or stimulus-responsiveness, extending their capabilities far beyond passive materials. At their core, nanomaterials serve as versatile scaffolds endowed with unique electronic, optical, magnetic, or catalytic properties. Coupling them with biological components, from enzymes and nucleic acids to cells and living organisms, creates functionalities unattainable by either component alone. The integration of robotic or actuation mechanisms adds a new dimension, enabling systems that can navigate, reconfigure, and execute sequential tasks in response to external stimuli or internal logic. The potential impact of hybrid nanobiotics spans a wide spectrum of fields, including targeted drug delivery and biosensing.
Leveraging our multidisciplinary expertise in microfluidics, nanomaterials, and biosensing, our group is uniquely poised to develop innovative hybrid nanobiotic platforms for intelligent diagnostics. For example, we recently reported a hybrid nanocomposite in which enzymes were incorporated into synthetic metal–organic framework (MOF) to enhance their stability and catalytic activity, vastly magnifying sensitivity for detecting cancer markers. Our long-term interest is to develop advanced hybrid nanobiotics to transform diagnostic biosensing, enabling intelligent detection of biomarkers in vivo or in vitro with unprecedented analytical performance and clinical utility.
Selected Publications:
- L. Zheng, N, Hart, Y. Zeng, “Micro-/Nanoscale Robotics for Chemical and Biological Sensing”, Lab on a Chip, 2023, 23, 3741-3767 (Invited Review).
- J. Shi, S. C. Barman, S. Cheng, Y. Zeng, “Metal-Organic Framework-Interfaced ELISA Probe Affords Ultrasensitive Detection of Extracellular Vesicle Biomarkers”, Journal of Materials Chemistry B, 2024, 12, 6342-6350.
- N. He, et al., “Nano Pom-poms Prepared Exosomes for Highly Specific Cancer Biomarker Detection”, Communications Biology (Nature), 2022, 5, 660.